Volume II ● Issue 1/2011 ● Pages 27–38
Paleoparasitological Findings in Medieval and Early Modern Archaeological Deposits from Hradební Street, Chrudim, Czech Republic
Lenka Bartošováa*, Oleg Ditrichb, Jaromír Benešc, Jan Frolíkd, Jan Musile
aFaculty of Science, University of South Bohemia, Branišovská 31, 370 05 České Budějovice, Czech Republic
bLaboratory of Veterinary and Medical Protistology, Institute of Parasitology, Branišovská 31, 370 05 České Budějovice, Czech Republic
cLaboratory of Archaeobotany and Palaeoecology, Faculty of Science, University of South Bohemia, Branišovská 31, 370 05 České Budějovice, Czech Republic
dInstitute of Archaeology, Academy of Sciences v.v.i., Letenská 4, 118 00 Prague, Czech Republic
eRegional Museum in Chrudim, Široká 86, 537 01 Chrudim, Czech Republic
Abstract
Extensive archaeological research including several environmental analyses was carried out in the historic centre of Chrudim in 2006. This article presents the results of the paleoparasitological investigation, which provided evidence of the level of hygiene and infestation of medieval and early modern populations (14th to 18th centuries). Organic settlements at the bottom of sewage dumps were especially rich sources of information on parasitic infestation. Five species of intestinal worms were identified with certainty: Trichuris trichiura, Ascaris lumbricoides, Toxocara canis/cati, Diphyllobothrium latum and Fasciola hepatica, and three others are considered likely: Hymenolepis nana, Enterobius vermicularis and Ancylostoma duodenale. Testing for the antigen GSA 65, evidence of Giardia lamblia, yielded positive results. The findings of the parasitological examination are evaluated in connection with the occurrence of parasites in humans, food quality and other health determinants of medieval populations.1
Article info
Article history:
Received: 27 July 2011
Accepted: 5 September 2011
Keywords:
paleoparasitology
medieval town
cesspit
helmints
hygiene
1. Introduction
Paleoparasitology is a young discipline that demonstrates the age-long relationship between humans and parasites and their spread in human populations and human migration in the past (Reinhard 1992, Gonçalves et al. 2003, Sianto et al. 2009). Aside from the analyses carried out by archaeobotanists, malacologists, palynologists, entomologists and other specialists, paleoparasitology has its own place in archaeological research and completes the picture of the investigated site (Bouchet et al. 2003b).
Palaeoparasitology has been developing as an adjunct of archaeology (Cox 2004) since the 1970s (Araújo et al. 1998), even though its foundation is associated with the earlier discovery of Schistosoma haematobium eggs in the kidneys of Egyptian mummies dated cca 1,250–1,000 BC (Stewart 1951, Araújo et al. 1998, Cox 2002, Bouchet et al. 2003b). Aside from mummified bodies or body parts, paleoparasitological examination also focuses on organic sediments and coprolites as potential sources (Reinhard 1992, Aspöck et al. 1999, da Rocha et al. 2006). Palaeoparasitological finds are mostly composed of eggs, less frequently of the larvae of intestinal parasites or of the chitin exosceletons of ectoparasites. Unlike other biological materials, such as pollen grains – which can remain preserved for a very long time in various conditions – parasitic worms only rarely create eggs with the same resistance to the external stressors. Some groups of parasites create structures resistant to fast decomposition, thin-walled eggs, as cysts of parasitic protozoas preserve very poorly (Araújo et al. 2000, Bouchet et al. 2003b). On the European continent, within Neolithic and later archaeological material we largely find Fasciola hepatica and Dicrocoelium dendriticum from the class Trematoda (Bouchet et al. 2003c, Dittmar, Teegen 2003).
Eggs of tapeworms (class Cestoda) such as Taenia spp. and Diphyllobothrium spp. can be found as far back as the Neolithic period. (Aspöck et al. 1999, Bouchet et al. 1999, 2003c, Gonçalves et al. 2003, Le Bailly et al. 2005, da Rocha et al. 2006). Infection by either of these two species is closely connected to certain dietary habits. The eggs of Taenia saginata and Taenia solium contaminate humans who consume inadequately cooked beef or pork (Aspöck et al. 1999, Bouchet et al. 2003c, Volf et al. 2007). One unresolved question remains whether the tapeworm eggs found represent T. saginata or T. solium and whether people were affected only with relatively harmless intestinal infections or suffered from cysticercosis (tissue infection) (Aspöck et al. 1999). Infected and inadequately cooked fish can be a source of Diphyllobothrium latum infection (Aspöck et al. 1999, Bouchet et al. 2003c, Volf et al. 2007).
Findings of eggs of the class Nematoda clarify the hygienic situation at a site, since the eggs of roundworms are indicators of faecal contamination. Their presence and volume are used to determine the function of various archaeological objects such as outhouses, cesspits, refuse pits, wells, ice boxes or agricultural objects such as silos (Bouchet et al. 2003c). It is assumed that Ascaris lumbricoides (a type of round worm) was a frequent parasite as early as prehistoric humans, but aside from the single site at Arcy-sur-Cure, Yonne in France dated cca 35,000 BC (Loreille, Bouchet 2003), all finds are dated to the Bronze Age (2,000–750 BC). Roundworm eggs are frequently found on medieval sites (Aspöck et al. 1999, Bouchet et al. 2003c, Gonçalves et al. 2003, da Rocha et al. 2006). Ascaris lumbricoides and Ascaris suum are impossible to distinguish by their egg morphoplogy and therefore roundworms are identified with the help of other finds from the archaeological context (Loreille, Bouchet 2003, Fernandes et al. 2005). The question of the origin of A. lumbricoides has been discussed for many years (Gonçalves et al. 2003, Loreille, Bouchet 2003). Toxocora canis was identified in a collapsed cave in France, dated between 500,000 and 300,000 BC (Bouchet et al. 2003a).
The eggs of Trichuris trichiura are found frequently because their envelope is very resistant (Šebela et al. 1990, Aspöck et al. 1999, Bouchet et al. 2003c, Gonçalves et al. 2003). This is also the only parasite found in Őtzi, the mummy found in the Alps (Aspöck et al. 1996, Hidalgo-Argüello et al. 2003). The presence of T. trichiura and/or A. lumbricoides) eggs proves very poor hygienic conditions and both of these species are frequently found in one host. Dogs are often infected by T. vulpis, and pigs by T. suis – either of which can, on rare occasions, also infect humans (Volf et al. 2007). Capillaria hepatica is an urban rodent parasite that is transferable to humans and other mammals (Jíra 1998, Volf et al. 2007). The presence of their highly ornamental “watermelon pattern” eggs must be evaluated in the context of the given site (Bouchet 1997, Dittmar, Teegen 2003, Bouchet et al. 2003c, Fernandes et al. 2005). True infection of these parasites in humans is very rare (Nabi et al. 2007). The presence of Enterobius vermicularis eggs is rather rare in archaeological materials because their walls are very thin (Fry, Moore 1969, Araújo, Ferreira 2000, Gonçalves et al. 2003). Ancylostoma duodenale is often called the “hookworm of the old world”. Due to human migration this parasite spread from its home in the Middle East to the Mediterranean and in the opposite direction to the south-east Asia, Indonesia and the south Pacific (Anderson 2000). It was thought that this species spread into the Americas after Columbus, but now is widely accepted (and supported by paleporasitological findings) the idea of pre-Colombian colonisation by this and other parasites via the Bering Land Bridge (Gonçalves et al. 2003, Cox 2002). This parasite is now common in subtropical regions and locations with similar climate such as mines, brick-kilns, and the well-known case of the Gothard tunnel construction site in the Alps (Peduzzi, Piffaretti 1983). This parasite was also identified in the Czech coal basin in 1904 (Jíra 1998). Its presence in the archaeological materials in Europe is documented for example by Šebela et al. 1990, Aspöck et al. 1999, Gonçalves et al. 2003).
Paleoparasitological finds of protozoan parasites are rare (Araújo et al. 1998, Bouchet et al. 2003c) and experiments have shown that cysts once dry are subsequently very difficult to identify (Gonçalves et al. 2002). However, it appears that antigens of parasitic protozoans can remain identifiable for a long time, even with the use of commercially available kits (Gonçalves et al. 2002, 2004).
Molecular methods have found their place in archaeological research, first in the amplification of human DNA from bones and from mummified tissues, and later also in the field of paleoparasitology (Guhl et al. 1997, Araújo et al. 1998). aDNA (ancient DNA) is frequently damaged by hydrolytical or oxidative agents, and as a result broken into small components. This reduces the possibility of amplifying DNA or obtaining long PCR products (Golenberg et al. 1996, Capelli et al. 2003, Cipollaro 2005). This topic has been recently elaborated by some paleoparasitologists (Loreille et al. 2001, Martinez et al. 2003, Iñiquez et al. 2006).
Extensive archaeological excavations were carried out in Hradební Street, in the town of Chrudim in 2006, in advance of residential and commercial development. Excavations revealed a complex of settlement objects with rich inner stratification dated to the late Hallstat period. The main period of occupation was between the 10th and 17th centuries. The objective of this article is to present the paleoparasitological analysis conducted. The results of this analysis provide an insight into the human-parasite relationship during the development of the city from medieval to early modern times. Research conducted in Chrudim is one of the first, large-scale, parasitological analyses in the Czech Republic.
2. Archaeological site
The Hradební Street excavation in Chrudim was an extensive and complex archaeological event. The site is the largest area ever excavated in the town centre (Frolík, Musil 2007, Frolík, Musil 2010). The excavation was conducted in two yards and a part of a garden. The oldest finds belong to a group of layers and pits with large amounts of ceramic shards associated with a late Hallstat period fort of the “Slezskoplatěnická culture” (cca 800–600 BC, Novák 2010). A fortified settlement existed in the 9th–10th centuries AD (Frolík, Sigl 1998), the area of which corresponded to the area of the later medieval town. Findings from the excavated area include traces of wooden buildings and fences in three horizons, the youngest of which can be attributed to what is known regionally as the Fortification period of the 10th century (middle of the early Medieval period). Subsequent horizons evidence continuous development throughout the 11th to 13th centuries (late phase of the early medieval period).
The development of the medieval town was initiated sometime before 1276. Today’s Hradební Street as well as many other parcels of land were measured out. In the 2nd half of the 13th century buildings were made of wood, and remains were discovered of two wooden buildings in the style of log cabins. But by the 14th century log houses made way for timber buildings built on stone bases. Chrudim was also impacted by an extensive fire sometime in the first third of the 14th century.
Cesspits and refuse pits provide outstanding evidence of the overall picture. The 10 cesspits examined were rounded or straight-edged with wood or stone linings. The 4 refuse pits found were unlined. Chronologically they cover the period from the late 13th and early 14th to the 18th centuries. Subsequent to the 30 Years War (1618–1648), the refuse pits were the only objects built within the area of archaeological excavation. Most cesspits are associated with the house at 14/I Hradební Street. A total of 7 cesspits were used from the late 13th and early 14th centuries (cesspit 928 lower part) until the 17th century (cesspits 927, 932, 938, 932 and 951).
All cesspits and refuse pits were examined in the light of their natural layers. The cesspits originally contained a soft infill, which gradually settled into characteristic layers with sediment edges extruding upwards along the sides. Some cesspits show evidence of a light wooden covering. The upper layers of fill were usually much younger, often with dates in the 18th to 19th centuries (e.g. cesspit 938, upper part). The detailed differentiation of individual sediments in the cesspits, and the analysis of a small part of the finds, showed that some cesspits were partially cleaned out and subsequently reused. Glass (Kozáková 2009, Kozáková et al. 2010) and ceramic analysis evidenced at least four horizons in cesspit 938 and three in cesspit 943. Some cesspits were filled once, in a very short period of time, and not used any further (cesspits 962, 951, and an older cesspit 928). These are generally older and date to the 14th century.
The finds from the cesspits are profile typical medieval household items. Finds included a wide spectrum of kitchen and table ware (e.g. more than 100 dishes). The presence of kitchen stove tiles is a refinement that arrived in Chrudim as early as the 14th century. The glass finds from the 13th to 17th century are especially rich. It seems that glass comprised a component of household goods in almost all Chrudim homes.
3. Materials and methods
The basic sampling strategy included the collection of 88 samples of sediment for archeobotanical and other environmental analyses, during field excavation. Sediment from archaeological contexts was stored in plastic bags. Samples for palaeoparasitological analysis were removed from these bags with rubber gloves, stored in resealable plastic bags, and immediately labelled with bag and layer number. Before analysis they were stored in paper boxes at laboratory temperature. The entire contents of each bag were emptied into a large Petri dish and thoroughly mixed. A pea size sample was then taken using a plastic spoon. An analysis for one sample from each bag was conducted using sediment and flotation methods.
3.1 Sedimentation AMS III
The solution for the AMS III sedimentation method contains 115.2 g (dry) Na2SO4, 540 ml HCl and 660 ml H2O, at the required density of 1.08. The Triton solution was prepared by mixing 16.5 ml of Triton X-100 (isooctylphenoxypolyethoxyphenol) with 33.5 ml H2O. After the initial preparation of the sample and centrifugal sedimentation with 440g, the supernatant was poured off and the sediment was examined.
3.2 Flotation by Kozák and Mágrová (KOMA)
The KOMA solution was composed of one part of the solution A, one part of the solution B and one part of glycerol. Solution A was prepared with 560 g ZnSO4 in 1000 ml of distilled water. Solution B was prepared from 920 g MgSO4 in 1000 ml of distilled water. After the preparation of the sample and double centrifugation with 440g (first in distilled water and subsequently in KOMA) a test tube was filled with KOMA. The mouth of the test tube was capped by a cover slide, allowing adhesion of the surface film, which was then transferred to a slide and examined under microscope.
3.3 Rehydration
During the laboratory process it was decided to first rehydrate the samples. About one quarter of the material was gradually examined this way and then compared the quality of samples with samples directly processed by sedimentation and flotation methods. One gram of material was always placed in the test tube, 10 ml of 0.5% Na3PO4 was added and the contents were thoroughly stirred. The test tubes were covered with aluminium foil and refrigerated at 5 degrees Celsius for one week. After this the samples were processed by sedimentation and flotation methods.
3.4 Microscope technique
Samples were investigated under an Olympus IX70 or BX51 microscope. Eggs were identified by their characteristic marks and morphometry (Ash, Orihel 2007).
3.5 Detection of antigens of protozoan parasites
3.5.1 Entamoeba histolytica
In attempts to detect the E. histolytica antigen we used a commercially available kit, which detects this antigen on the basis of a form of enzyme-linked immunosorbent assay (known as “sandwich ELISA”). Aside from controls included in the kit, each experiment also used an additional negative control – parasite free soil. A sample of a parasite positive stool of an Entamoeba infected patient for positive control was not available. The samples were always examined in triplets. The result of each test was only valid when positive and negative controls of the kit reached an absorbency (A) value given by manufacter. A sample is considered reactive when the value of A reaches more than fixed cut-off A value. The test was considered valid when the value of A of the negative sample (parasite free soil) was not higher than the value of the negative sample from the kit. The measurements were carried out on an ELISA reader at 450 nm. The final result is the arithmetic average of the measured A values of the sample triplet. Outliers (values that differ more than 1.5x) are not included.
3.5.2 Giardia intestinalis
In attempt to detect the G. intestinalis antigen we used a simple commercially available ELISA kit. Aside from controls included in the kit, each experiment also used a negative control (parasite free soil) and positive control (stool sample from parasite positive patient). The samples were always examined in triplets. The test results were valid only when positive and negative controls reached an absorbency (A) value given by manufacter. The cut-off is calculated by adding the concrete A value to the arithmetic average of A values of the negative controls. For a sample to be considered reactive, its A value must be higher than the established cut-off value. The test is considered valid when the A value of the negative control sample (parasite free soil) does not exceed the cut-off value. The measurements were carried out on the ELISA reader at 450 nm. The final result is the arithmetic average of the measured A values of the sample triplet. Outliers (values that differ more than 1.5×) are not included.
4. Results
Organic sediments from cesspits at Chrudim were exceptionally rich in parasite finds, not only in terms of volume, but also in the variety of species identified. There were five species of intestinal worms from Hradební street: Trichuris trichiura (Figure 7), Ascaris lumbricoides (Figure 4 – unfertilized egg, Figure 5 – fertilized egg), Toxocara canis/cati (Figure 6), Diphyllobothrium latum (Figure 2) and Fasciola hepatica (Figure 1). Another three species were identified with high probability: Hymenolepis nana (Figure 3), Enterobius vermicularis (Figure 8) and Ancylostoma duodenale (Figure 9). From 88 collected samples 72 were from various cesspits, the rest came from various archaeological contexts (cellars, settlement layers, waste pits, granary and tomb – Table 1). Samples from cesspits provided a total of 51 positive samples. The highest number of eggs in one sample was 125 (T. trichiura, sample no. 145). Cesspits provided the most interesting results, as can be seen from the summary Figure 10. See individual tables of cesspits 927, 928, 932, 938 (Tables 2–5). The highest number of samples was taken and examined from the cesspit 938 – in the Figure 11 can be seen a spectrum of parasite findings. Layers of this cesspit were chosen for ELISA testing. All results from Entamoeba histolytica ELISA testing were negative. A reactive result, with GSA 65 antigen characteristic for Giardia lamblia, was obtained here 7 times. The interpretation of the results is difficult because of the small number of tested samples.
5. Discussion
The spectrum of parasites in Chrudim is in accordance with the findings at other similar sites. Trichuris, Ascaris and possibly Ancylostoma are present everywhere in places with poor sanitary conditions. This is certainly true in the medieval and early modern periods, when refuse was widely deposited, in refuse pits, in the yard, and in front of the house in the street. Also, there were often several generations in high density living arrangements in earthen-houses as well as in later log or brick houses. People usually had very few clothes and poor people often only one set of clothes. Undergarments were worn at night as well as during the day. People washed rarely, partially due to the relative lack of water (only richer people had their own wells, and most people had to carry water from a common well), and partially because there was a common belief that water was harmful and that it weakened the human body (Vondruška, 2007). All these aspects contributed to the population being rife with parasites.
From the point of view of the relationship between the spectrum of parasite species and the dating of the individual layers (Table 6) we come to the conclusion that during the entire examined period the species associated with orofecal transfer predominate. These include T. trichiura which appeared at highest quantity, and A. lumbricoides. A decrease in the number of species transferred in the orofecal way occurs at the turn of the 16th and 17th century, when zoonotic species begin to predominate in the spectrum of identified pathogens, e.g. Diphyllobothrium latum and Fasciola hepatica. This is possibly due to the richer diet. A significant shift in the quantity of orofecal transmitted species occurs in the 17th and 18th centuries, where we note a decrease in the occurrence of T. trichiura and an increase of A. lumbricoides. The species Toxocara canis/cati, Diphyllobothrium latum and Fasciola hepatica comprise less than 1% of the samples, and in fact are statistically insignificant. However, in connection with the archaeozoological analysis they do help to complete the picture of dietary habits at this site and give us an idea of the spectrum of animals that may have been present.
The quantitative analysis suggests that Trichuris trichiura is the dominant species, though we must be cautious with this conclusion, because the species have different life-cycles and the eggs have different predispositions to decomposition. For this reason, finding thin walled eggs is rare, e.g. Enterobius species, but even thick-walled eggs have differing levels of preservation. Even though some authors assert that the eggs of T. trichiura are decomposed by moulds, when in outhouses (Reinhard et al. 1992), the eggs of T. trichiura were found to be the best preserved species in the cesspits of Chrudim.
Ascaris lumbricoides is the second most frequent parasite in Chrudim. Together with T. trichiura they represent the most frequently found species, and evidence frequent orofecal parasite transfer as a result of poor hygiene right up to the early 18th century. Eggs of Toxocara canis/cati, are also relatively frequent, evidence of the presence of breed or free-roaming dogs and cats on the site. This is also evidenced by several complete cat skeletons (that probably died of natural causes) (Baloghová 2010).
It is surprising that there were no finds of Capillaria, a normally common find (Bouchet 1997, Dittmar, Teegen 2003, Fernandes et al. 2005) related to the ubiquitous rodents in the medieval period. Rodents were also detected in cesspits 938 and 973 by archaeozoological analysis (Baloghová 2010). Capillaria eggs may have occurred in cesspits only sporadically since rodents would transfer excrements with the eggs more frequently on the free surface around the houses where they were likely to get destroyed by weather conditions.
The identification of a hookworm egg (Ancylostoma duodenale) would be a unique find, and might be confirmed by more detailed research and molecular analysis. Despite Šebela’s report of a hookworm egg from a Bronze Age site near Hulín (Šebela et al. 1990, Aspöck et al. 1999), some authors remain sceptical about its occurrence in Moravia 3.500 years ago. In any case, research on other sites should be carried out in order to clarify whether Ancylostoma duodenale was locally present in Moravia, since at the present time this parasite is endemic to tropical and subtropical climates.
The discovery of Diphyllobothrium latum eggs of the class Cestoda shows that the Chrudim population had access to fish. This is evident from finds in cesspits 938 and 973 (Baloghová 2010). Even though these egg finds were not frequent (a total of 14 in 4 layers), it is possible that they are a proof of true human infection. The eggs of D. latum are thin walled and thus were probably preserved in limited numbers. It is also possible that infected leftovers were fed uncooked to domestic animals, which could also become a source of eggs. However, we are disinclined to accept this hypothesis because food was scarce during the entire medieval period and people maximized intake. Fish leftovers would more likely be used for soup base than fed to animals. From the same class comes a unique find of an egg reminiscent of Hymenolepis nana. It is again important to consider that the source of this type of egg can be not only humans but also rodents.
The complete absence of Taenia sp. comes as a surprise. The reason cannot be limited access to meat because the town population included relatively rich burghers. We believe that consumption of partially raw meat was not as prevalent in Bohemian society as it was among French burghers and aristocrats (for example Bouchet et al. 2003c, da Rocha et al. 2006). If Chrudim’s population preferred baked or properly cooked meat then they killed the infectious stages of tapeworms by heat and avoided infection. The absence of Taenia sp. was also noted during the parasitological examination of a large number of samples from an archaeological excavation on Narodní Avenue in Prague (Myšková 2011).
Several eggs of Fasciola found at the site are due its low number considered transitory. This means that they passed unchanged through the digestive track of people after ingestion of infected livers. It is also possible that these eggs came directly from domestic animals, however, animal pens were not confirmed at this site.
The discovery of two pin worm eggs is a rare find. The thin-walled eggs of Enterobius vermicularis are very difficult to preserve and to this day there are very few documented finds in Europe (Gonçalves et al. 2003). These eggs were found in the layer with clay like texture, which is what most likely made their preservation possible. The literature states that besides freezing and extremely dry conditions, moist anaerobic environment is ideal for preserving the structure of parasite eggs (Araújo et al. 2000, Bouchet et al. 2003c, da Rocha et al. 2006).
The results of the ELISA test cannot be evaluated as unequivocally as those of direct microscopy. Because no cysts of parasitic protozoa were found in the samples, the ELISA method was not a confirmatory method, but was used to try to determine whether the material contained antigens of parasitic protozoa. As with modern patient samples, the cysts (and antigens) in the stool are not released continuously but are distributed in uneven clusters (García et al. 2000, Gonçalves et al. 2002). We have to take this fact into consideration when examining archaeological samples. This can help explain why in one sample the first test detected an antigen and in a subsequent test the same sample was negative. This result needs to be evaluated with certain scepticism, because the theme of detection of antigens of parasitic protozoa in archaeological material is still relatively new. Samples are considered probably positive if at least two of three tests of one sample were positive. Samples with one test positive should be considered only potentially positive. Confidence that a positive result will mean detection of particular antigen and not only non-specific reaction was increased by the inclusion of a proper negative control sample. The incubation of a garden soil sample always showed A values that were lower than established cut-off value.
The resistance of the antigen structure GSA 65, the main surface antigen of Giardia intestinalis, was tested by Rosoff in 1986. He found that GSA 65 has unchanged immunoreactivity after 10 min of boiling, after proteolytic dissolution (by trypsin, chymotrypsin, proteasis), formalin fixation, as well as after long-term storage (6 months at 4 as well as –20 degrees Celsius, Rosoff, Stibbs 1986). Identification and characterization of GSA 65 showed its applicability in the ELISA coprodiagnostic system. In the case of Entamoeba histolytica the antigen is Gal/GalNAc lectin. With the use of a monoclonal antibody it is possible to differentiate E. histolytica from non-pathogenic E. dispar. These tests together with recent studies by the Goncalves’ paleoparasitological research team suggest that some antigen structures can be preserved in archaeological material (Gonçalves et al. 2002, 2004). Despite this, we believe that in the near future the number one method of parasite detection will be the isolation and detection of DNA. In the case of positive results the DNA method allows sequencing of the final product and thus unequivocally identifies particular species.
Paleoparasitological studies in historic Chrudim have contributed substantially to our understanding of hygiene and the lifestyle of the population in the medieval and early modern periods. One important tasks of parasitology in this area is the comparison of the parasite situation between individual towns or villages, as well as in various types of social environments and its changing in time.
Acknowledgement
The work was supported by a grant from the Faculty of Science, University of South Bohemia (MSM6007665801 & GAJU 138/2010/P).
References
ALLISON, M. J., BERGMAN, T., GERSZTEN, E. 1999: Further studies on fecal parasites in antiquity, Americal Journal of Clinical Pathology 112, 605–609.
ANDERSON, R. C. 2000: Nematode parasites of vertebrates: their development and transmission. CABI Publishing, Oxon, 2nd edition.
ARAÚJO, A., REINHARD, K., BASTOS, O. M., COSTA, L. C., PIRMEZ, C., IÑIGUEZ, A., VICENTE, A. C., MOREL, C. M., FERREIRA, L. F. 1998: Invited rewiew paleoparasitology: Perspectives with new techniques, Revista do Instituto de Medicina Tropical de São Paulo 40 (6), 371–376.
ARAÚJO, A., FERREIRA, L. F. 2000: Paleoparasitology and the antiquity o human host-parasite relationship, Memórias do Instituto Oswaldo Cruz, Rio de Janeiro 95 (Suppl. I): 89–93.
ARAÚJO, A., JANSEN, A. M., BOUCHET, F., REINHARD, K., FERREIRA, L. F. 2003: Parasitism, the diversity of life and paleoparasitology, Memórias do Instituto Oswaldo Cruz, Rio de Janeiro 98 (Suppl. I): 5–11.
ASH, L. R., ORIHEL, T. C. 2007: Atlas of human parasitology. Chicago, American Society for Clinical Pathology Press, 5th edition.
ASPÖCK, H., AUER, H., PICHER, O. 1996: Trichuris trichiura eggs in the Neolithic glacier mummy from the Alps, Parasitology Today 12 (7), 255–256.
ASPÖCK, H., AUER, H., PICHER, O. 1999: Parasites and parasitic diseases in prehistoric human populations in central Europe, Helminthologia 36 (3), 139–145.
LeBAILLY, M., LUEZINGER, U., SCHLICHTHERLE, H., BOUCHET, F. 2005: Diphyllobothrium: Neolithic parasite? Journal of Parasitology 91 (4): 957–959.
BALOGHOVÁ, R. 2010: Archeozoologie tří vrcholně středověkých parcel v Chrudimi – Hradební ulici. Archeozoology of three high-medieval plots in Chrudim – Hradební street. MS. Master diploma thesis. Deposited: Faculty of Science, The University of South Bohemia, České Budějovice.
BARTOŠOVÁ, L., 2009: Paleoparazitologická analýza organických sedimentů archeologického naleziště v Chrudimi. Paleoparasitological analysis in organic sediments on archeological locality in Chrudim. MS. Master diploma thesis. Deposited: Faculty of Science, The University of South Bohemia, České Budějovice.
BOUCHET, F. 1997: Intestinal capillariasis in neolithic inhabitants of Chalain (Jura, France), Lancet 349, 256.
BOUCHET, F., LEFÉVRE, C., WEST, D., CORBETT, D. 1999: First paleoparasitological analysis of a midden in the Aleutian islands (Alaska): results nas limits, Journal of Parasitology 85 (2), 369–372.
BOUCHET, F., ARAÚJO, A., HARTER, S., CHAVES, S. M., DUARTE, A. N., MONNIER, J. L., FERREIRA, L. F. 2003a: Toxocara canis (Werner, 1782) eggs in the pleistocene site of Menez-Dregan, France (300,000–500,000 years before present), Memórias do Instituto Oswaldo Cruz, Rio de Janeiro 98 (Suppl. I), 137–139.
BOUCHET, F., GUIDON, N., DITTMAR, K., HARTER, S., FERREIRA, L. F., CHAVES, S. M., REINHARD, K., ARAÚJO, A. 2003b: Parasite remains in archeological sites, Memórias do Instituto Oswaldo Cruz, Rio de Janeiro 98 (Suppl. I): 47–52.
BOUCHET, F., HARTER, S., Le BAILLY, M. 2003c: The state of the art of paleoparasitological research in the Old World, Memórias do Instituto Oswaldo Cruz, Rio de Janeiro 98 (Suppl. I): 95–101.
CAPELLI, C., TSCHENTSCHER, F., PASCALI, V. L. 2003: “Ancient” protocols or the crime scene? Similarities and differences between forensic genetics and ancient DNA analysis, Forensic Science International 131: 59–64.
CIPOLLARO, M., GALDERISI, U., di BERNARDO, G. 2005: Ancient DNA as a multidisciplinary experience, Journal of cellular physiology 202: 315–322.
COX, F. E. G. 2002: History of human parasitology, Clinical Microbiology Reviews, Oct., 595–612.
COX, F. E. G. 2004: History of human parasitic diseases, Infectious Disease Clinics of North America 18: 171–188.
CRAIG, P., ITO, A. 2007: Intestinal cestodes, Current Opinion in Infectious Diseases 20: 524–532.
DITTMAR, K., TEEGEN, W. R. 2003: The presence of Fasciola hepatica (liver-fluke) in humans and cattle from a 4,500 year old archeological site in the Saale-Unstrut valley, Germany, Memórias do Instituto Oswaldo Cruz, Rio de Janeiro 98 (Suppl. I), 141–143.
FAULKNER, C. T., SHARON, P., JOHNSON, S. S. 1989: Prehistoric parasitism in Tennessee: evidence from the analysis of desiccated fecal material collected from Big Bone cave, Van Burden, Tennessee, Journal of Parasitology 75, 461–463.
FERNANDES, A., FERREIRA, L. F., GONÇALVES, M. L. C., BOUCHET, F., KLEIN, C. H., IGUCHI, T., SIANTO, L., ARAÚJO, A. 2005: Intestinal parasite analysis in organic sediments collected from a 16th century Belgian archeological site, Cadernos de Saúde Pública, Rio de Janeiro 21(1): 329–332.
FROLÍK, J., SIGL, J. 1998: Chrudim v pravěku a středověku. Obrazy každodenního života. Okresní muzeum Chrudim, Chrudim.
FROLÍK, J., MUSIL, J. 2007: Záchranné archeologické výzkumy v Chrudimi v roce 2006, Archeologické výzkumy v Čechách 2006. Zprávy České společnosti archeologické, Suppl. 68: 46–48.
Frolík, J., Musil, J. 2010: Záchranný archeologický výzkum v Hradební ulici v roce 2006, Chrudimský vlastivědný sborník 14, 3–28.
FRY, G. F., MOORE, J. G. 1969: Enterobius vermicularis: 10000-year-old human infection, Science 166, 1620.
GARCÍA, L. S., SHIMIZU, R. Y., BERNARD, C. N. 2000: Detection of Giardia lamblia, Entamoeba histolytica/dispar, and Cryptosporidium parvum antigens in human fecal specimens using the Triage parasite panel enzyme immunoassay, Journal of Clinical Microbiology 39 (9), 3337–3340.
GOLENBERG, E. M., BICKEL, A., WEIHS, P. 1996: Effect of highly fragmented DNA to PCR, Nucleic Acids Research 24 (24), 5026–5033.
GONCALVES, M. L. C., ARAÚJO, A., DUARTE, R., da SILVA, J. P., REINHARD, K., BOUCHET, F., FERREIRA, L. F. 2002: Detection of Giardia duodenalis antigen in coprolites using a commercially available enzyme-linked immunosorbent assay, Transactions of the Royal Society of Tropical Medicine and Hygiene 96, 640–643.
GONCALVES, M. L. C., ARAÚJO, A., FERREIRA, L. F. 2003: Human intestinal parasites in the past: new findings and a review, Memórias do Instituto Oswaldo Cruz, Rio de Janeiro 98 (Suppl. I), 103–118.
GONCALVES, M. L. C., da SILVA, V. L., de ANDRADE, C. M., REINHARD, K., da ROCHA, G. Ch., Le BAILLY, M., BOUCHET, F., FERREIRA, L. F., ARAÚJO, A. 2004: Amoebiasis distribution in the past: first steps using an immunoassay technique, Transactions of the Royal Society of Tropical Medicine and Hygiene 98, 88–91.
GUHL, F., JARAMILLO, C., YOCKTENG, R., VALLEJO, G. A., ARROYO, F. C. 1997: Trypanosoma cruzi DNA in human mummies, Lancet 349, 1370.
HIDALGO-ARGÜELLO, M. R., BAÑOS, N. D., GRANDES, J. F., MARCOS, E. P. 2003: Parasitological analysis of Leonese royalty from Collegiate-basilica of St. Isidoro, León (Spain): Helminths, Protozoa, and Mites, Journal of Parasitology 89 (4), 738–743.
IÑIGUEZ, A. M., REINHARD, K., GONCALVES, M. L. C., FERREIRA, L. F., ARAÚJO, A., VICENTE, A. C. P. 2006: SL1 RNA gene recovery from Enterobius vermicularis ancient DNA in pre-Columbian human coprolites, International Journal for Parasitology 36, 1419–1425.
JÍRA, J. 1998: Lékařská helmintologie. Galén, Praha.
KODÝDKOVÁ, K. (2009): Analýza rostlinných makrozbytků ze středověké odpadní jímky v Chrudimi. The archeobotanical research of the medieval cesspit in Chrudim (Czech Republic). MS. Bachelor diploma thesis. Deposited: Faculty of Science, University of South Bohemia, České Budějovice.
KOZÁKOVÁ, R. 2009: Restaurování archeologických skel z Chrudimi a možnosti konzervace metodou sol-gel. MS. Bachelor diploma thesis. Deposited: University of Chemistry and Technology in Prague. Faculty of Chemical Technology. Institute of Glass and Ceramics, Prague.
Kozáková, R., Klikarová, L., Frolík, J. 2010: Bohatý soubor skla v Chrudimi – Hradební ulice, Chrudimský vlastivědný sborník 14, 129–166.
LOREILLE, O., ROUMAT, E., VERNEAU, O., BOUCHET, F., HÄNNI, C. 2001: Ancient DNA from Ascaris: extraction, amplification and sequences from eggs collected in coprolites, International Journal for Parasitology 31, 1101–1106.
LOREILLE, O., BOUCHET, F. 2003: Evolution of Ascaris in humans and pigs: a multi-disciplinary approach, Memórias do Instituto Oswaldo Cruz, Rio de Janeiro 98 (Suppl. I), 39–46.
MARTINES, E. M., CORREIA, J. A. S., VILLELA, E. V. 2003: Random amplified polymorphic DNA analysis of DNA extracted from Trichuris trichiura (Linnaeus, 1771) eggs and its prospective application to paleoparasitological studies, Memórias do Instituto Oswaldo Cruz, Rio de Janeiro 98 (Suppl. I), 59–62.
MYŠKOVÁ, E. 2011: Paleoparazitologická analýza organických sedimentů archeologického naleziště na Národní třídě, Praha. Paleoparasitological analysis of organic sediments on archeological locality in Národní třída, Prague. MS. Bachelor diploma thesis. Deposited: Faculty of Science, The University of South Bohemia, České Budějovice.
NABI, F., PALAHA, H. K., SEKHSARIA, D., CHIATALE, A. 2007: Capillaria hepatica infestation, Indian Pediatrics 44, 781–782.
Novák, M. 2010: Pravěké osídlení Chrudimi – Hradební ulice, Chrudimský vlastivědný sborník 14, 29–67.
PEDUZZI, R., PIFFARETTI, J. C. 1983: Ancylostoma duodenale and the Saint Gothard anaemia, British Medical Journal 287, 1942–1945.
REINHARD, K. J. 1992: Parasitology as an interpretative tool in archaeology, American Antiquity 57 (2), 231–245.
da ROCHA, G. Ch., HARTER-LAILHEUGUE, S., Le BAILLY, M., ARAÚJO, A., FERREIRA, L. F., da SERRA-FREIRE, N. M., BOUCHET, F. 2006: Paleoparasitological remains revealed by seven historic contexts from “Place d’Armes”, Namur, Belgium, Memórias do Instituto Oswaldo Cruz, Rio de Janeiro 101 (Suppl. II), 43–52.
ROSOFF, J. D., STIBBS, H. H. 1986: Physical and chemical characterisation of Giardia lamblia-specific antigen useful in the coprodiagnosis of giardiasis. Journal of Clinical Microbiology 24 (6), 1079–1083.
SIANTO, L., CHAME, M., SILVA, C. S. P., GONCALVES, M. L. C., REINHARD, K., FUGASSA, M., ARAÚJO, A. 2009: Animal helminths in human archeological remains: A review of zoonoses in the past, Revista do Instituto de Medicina Tropical de São Paulo 51 (3), 119–130.
STEWART, I. E. 1951: Helmints in history, The Scientific Monthly 72 (6), 345–352.
ŠEBELA, L., VOJTKOVÁ, L., VOJTEK, J. 1990: Intestinal parasites in man of old bronze age, Anthropologie 28 (1), 105–107.
VOLF, P., HORÁK, P. et al. 2007: Paraziti a jejich biologie. Nakladatelství TRITON, Praha.
VONDRUŠKA, V. 2007: Intimní historie. Moba, Brno.
*Corresponding author, e-mail: lenabart@atlas.cz
1The excavation was carried out by the Institute of Archaeology in Prague and by the Regional Museum in Chrudim. An extensive analysis of the samples is at present being carried out, in cooperation with the University of South Bohemia in České Budějovice, in the areas of archaeobotany, archaeozoology and palaeoparasitology (Kodýdková 2009, Baloghová 2010, Bartošová 2009). This article is largely derived from the dissertation work of L. Bartošová.
Table 1. Examined object on archaeological site and number of samples.
Object No. samples No. positive samples
cesspit 927 9 4
cesspit 928 9 6
cesspit 932 8 1
cesspit 938 33 29
cesspit 943 4 3
cesspit 951 3 2
cesspit 962 4 4
cesspit 973 2 2
tomb 10th cent. 2 0
hollow under the wall 1 0
granary 10th cent. 1 0
settlement layer B5 1 0
settlement layer C7 1 0
settlement layer N6 1 0
settlement layer H6 1 0
waste pit 17th cent. 2 2
waste pit 18th cent. 4 0
cellar 14th cent. 1 0
cellar 17th cent. 1 1
Summary 88 54
Table 2. Findings in cesspit No. 927.
Sample Date Finds
Sedimentation Flotation
TR TR
5 17th cent.
6 17th/18th cent. 18
11 late 15th cent./1st half of 16th cent. 3
12 late 15th cent./1st half of 16th cent.
14 1st half of 15th cent.
21 14th/15th cent.
27 without date 12
30 14th/15th cent. 4 1
35 1st half of 14th cent.

Figure 1. Fasciola hepatica, sample No. 48, flotation. Operculum on the left pole, inside remains of the original content and air bell.
Figure 2. Diphyllobothrium latum, sample No. 48, sedimentation. Operculum on the left side, on the opposite side characteristic “knob”.
Figure 3. Presumably Hymenolepis nana, sample No. 198, flotation. Destroyed egg, could be distinguished two hooks inside.
Figure 4. Ascaris lumbricoides, unfertile egg, sample No. 259, flotation. Elongated, almost completely decorticated egg, mineralized content.
Figure 5. Ascaris lumbricoides, fertile egg, sample No. 246, sedimentation. Almost round egg, thich outher mammilliated coat, without mineralization.
Figure 6. Toxocara canis/cati, sample No. 199, flotation. Detail of the characteristic deep cup-like pits covering the surface of the shell (golf ball); mineralized content.
Figure 7. Trichuris trichiura, sample No. 182, flotation. Characteristic barrel-shape eggs, with bipolar plugs, granulation of the content.
Figure 8. Enterobius vermicularis, sample No. 251, flotation. Lightly destroyed egg, could be seen double smooth shell and asymetry in the shape of the egg; mineralized content.
Figure 9. Presumably Ancylostoma duodenale, sample No. 90, flotation. Clevage underneath suggests it could be the hookworm egg.
Figure 10. Spectrum of parasite in particular cesspits.
Figure 11. Spectrum of parasite in cesspit 938.
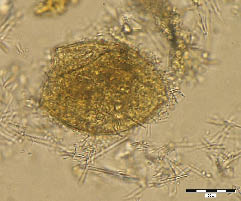

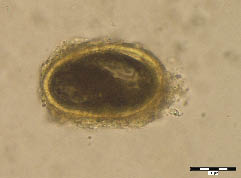
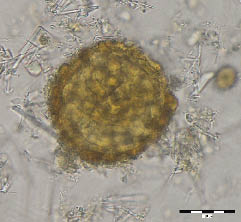
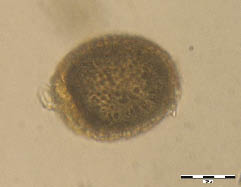


Table 3. Findings in cesspit No. 928.
Sample Date Finds Rehydration Finds
Sedimentation Flotation Sedimentation Flotation
TR AS TA DB TR AS TA DB TR AS TA DB TR AS TA DB
220 13th–14th cent.
232 13th–14th cent.
233 13th–14th cent.
234 13th–14th cent. 20 48 1 3 X 9 13 5 1
244 13th–14th cent. 4 3 39 6 X 5 1 1 2
246 13th–14th cent. 20 6 87 4 X
251 late 13th cent. 6 10 X 4 5 6 3
252 late 13th cent. 10 30 1 X 6 10 5
254 late 13th cent. 8 50

Table 4. Findings in cesspit No. 932.
Sample Date Finds Rehydration Finds
Sedimentation Flotation Sedimentation Flotation
TR TR TR TR
250 14th–15th cent. 2 2
253 18th cent.
255 14th cent.
261 14th cent.
262 14th cent. X
269 14th cent.
290 14th cent.
291 14th cent.


Table 5. Findings in cesspit No. 938.
Sample Date Finds Rehydration Finds ELISA Result
Sedimentation Flotation Sedimentation Flotation
TR AS DB TR AS TA DB FS TR AS TA TR AS TA
7 18th cent. 1 4 1 X 7 X neg.
13 17th cent. 2 1 X X neg.
45 13th-14th cent. 2 8 2 X 12 2 X neg.
48 16th/17th cent. 2 6 2 X 2 2
49 16th/17th cent.
51 16th/17th cent. X
60 15th cent. 4
61 15th/16th cent. X neg.
62 14th cent. 2
72 2nd half of 15th cent. 12 14 X 2× reactive
84 2nd half of 15th cent. 6 13 X 2× reactive
89 2nd half of 15th cent. 8 10
90 late 14th cent. 6 11 X 17 1 1 1 X neg.
95 15th cent. 17 2 19 X 5 32
101 15th cent. 6 46 1 X 14 1 21
117 late 14th cent./1st half of 15th cent. 20 46 X neg.
123 late 14th cent./1st half of 15th cent. 8 2 59 2 4 2 X 11 2 58
137 late 14th cent./1st half of 15th cent. 12 57 X 1× reactive
145 late 14th cent./1st half of 15th cent. 16 125 X 29 83 X neg.
150 late 14th cent./1st half of 15th cent. 26 30
151 late 14th cent./1st half of 15th cent. 12 2 30 X neg.
152 late 14th cent./1st half of 15th cent. 17 51 1 X 2 2 X neg.
162 late 14th cent./1st half of 15th cent. 15 81 X neg.
174 late 14th cent./1st half of 15th cent. 2 119 6 X 21 3 93 X 1× reactive
182 14th cent. 9 36
193 14th cent. 99
195 14th cent. 6 58 2 X 3 15
198 14th cent. 3 18 X 9 14 2 X neg.
206 14th cent. 8 57 X 7 3 X neg.
207 14th cent. 3 1 16 X 5 X 1× reactive
211 14th cent. 3 2 20 X neg.
216 strat. 14th cent. 10 X 8 X neg.
TR – Trichuris trichiura; AS – Ascaris lumbricoides; TA – Toxocara canis/cati; DB – Diphyllobothrium latum; FS – Fasciola hepatica; X – procedure was performed.
Table 6. Relationship between the spectrum of parasite species and the dating of the individual layers.
Period Samples Trichuris Ascaris Toxocara Diphyllobothrium Fasciola Giardia
trichiura lumbricoides canis/cati latum hepatica lamblia
(ELISA)
13th–14th cent. cesspit 928: 220, 232, 88.19% (381) 10.42% (45) 0.93% (4) 0.46% (2)
233, 234, 244, 246, 251,
252, 254
14th cent. cesspit 927: 35, 62; 98.33% (412) 1.19% (5) 0.48% (2) 1× reactive
cesspit 932: 255, 261,
262, 269, 290, 291;
cesspit 938: 182, 193,
195, 198, 206, 207,
211, 216
14th/15th cent. cesspit 927: 21, 30; 97.63% (1069) 1.55% (17) 0.27% (3) 0.37% (4) 0.18% (2) 2× reactive
cesspit 932: 250;
cesspit 938: 90, 117, 123,
137, 145, 150, 151, 152,
162, 174
15th cent. cesspit 927: 14; 97.62% (164) 2.38% (4)
cesspit 938: 60, 95, 101
15th/16th cent. cesspit 927: 11, 12; 100% (66) 4× reactive
cesspit 938: 51, 61, 72,
84, 89
16th/17th cent. cesspit 938: 45, 48, 49 85% (34) 5% (2) 5% (2) 5% (2)
17th–18th cent. cesspit 927: 5, 6, 13; 61.77% (21) 35.29 % (12) 2.94% (1)
cesspit 932: 253; cesspit 938: 7